+86-13708084407
- All
- Product Name
- Product Keyword
- Product Model
- Product Summary
- Product Description
- Multi Field Search
- All
- Product Name
- Product Keyword
- Product Model
- Product Summary
- Product Description
- Multi Field Search
Views: 0 Author: Nicolas Joubert; Alain Beck;Charles Dumontet;Caroline Denevault Publish Time: 2021-11-05 Origin: National Library of Medicine








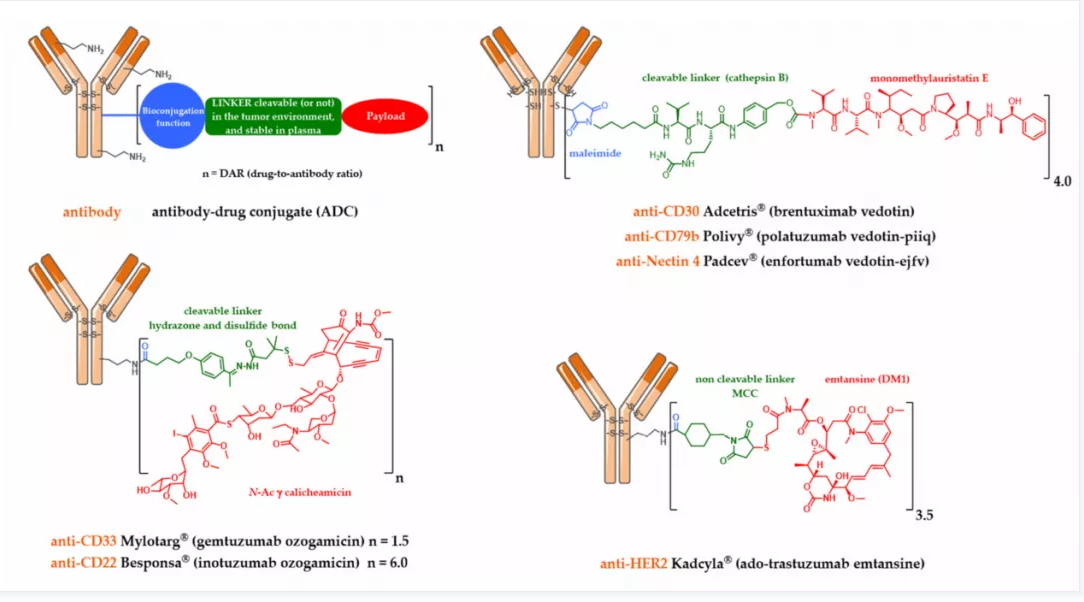
An armed antibody (antibody-drug conjugate or ADC) is a vectorized chemotherapy, which results from the grafting of a cytotoxic agent onto a monoclonal antibody via a judiciously constructed spacer arm. ADCs have made considerable progress in 10 years. While in 2009 only gemtuzumab ozogamicin (Mylotarg®) was used clinically, in 2020, 9 Food and Drug Administration (FDA)-approved ADCs are available, and more than 80 others are in active clinical studies. This review will focus on FDA-approved and late-stage ADCs, their limitations including their toxicity and associated resistance mechanisms, as well as new emerging strategies to address these issues and attempt to widen their therapeutic window. Finally, we will discuss their combination with conventional chemotherapy or checkpoint inhibitors, and their design for applications beyond oncology, to make ADCs the magic bullet that Paul Ehrlich dreamed of.
Keywords: ADC; antibody–drug conjugate; bioconjugation; cancer; combination therapies; linker; payload; resistance.
Alain Beck is an employee of Pierre Fabre, Charles Dumontet has received research funding from Roche and is a founder of Mablinks. All authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.